SAREMCO Dental AG Sets New Standard with Likely World’s First MDR Certification for Dental Composites
SAREMCO Dental AG, an independent Swiss company in the dental industry, has likely become the world’s first company to receive certification for a light-curing dental filling material – saremco els composite – under the new Medical Device Regulation (MDR EU 2017/745). This certification further demonstrates SAREMCO Dental’s leadership position in the field of light-curing dental materials.
The MDR includes a range of unprecedented new requirements to ensure the safety and quality of medical devices. MDR certification of a dental filling material means that the product has undergone extensive testing and quality controls. This includes evaluating the material’s biocompatibility, ensuring safety of the manufacturing process, and confirming the reliable function of the material under various conditions. By choosing an MDR-certified filling material, patients and dentists are assured that the product meets the latest and strictest safety requirements, significantly reducing the risk of complications and offering long-term health benefits.
saremco els composite has been used worldwide for over 20 years and has proven its clinical safety and reliability. It is characterized by a HEMA- and TEGDMA-free composition that reduces allergenic potential, as well as extremely low shrinkage stress, minimizing the risk of marginal gaps. With a broad, vibrant, and stable color palette, including four unique bleach shades and very low water absorption, the product offers optimal properties.
More about saremco els composite
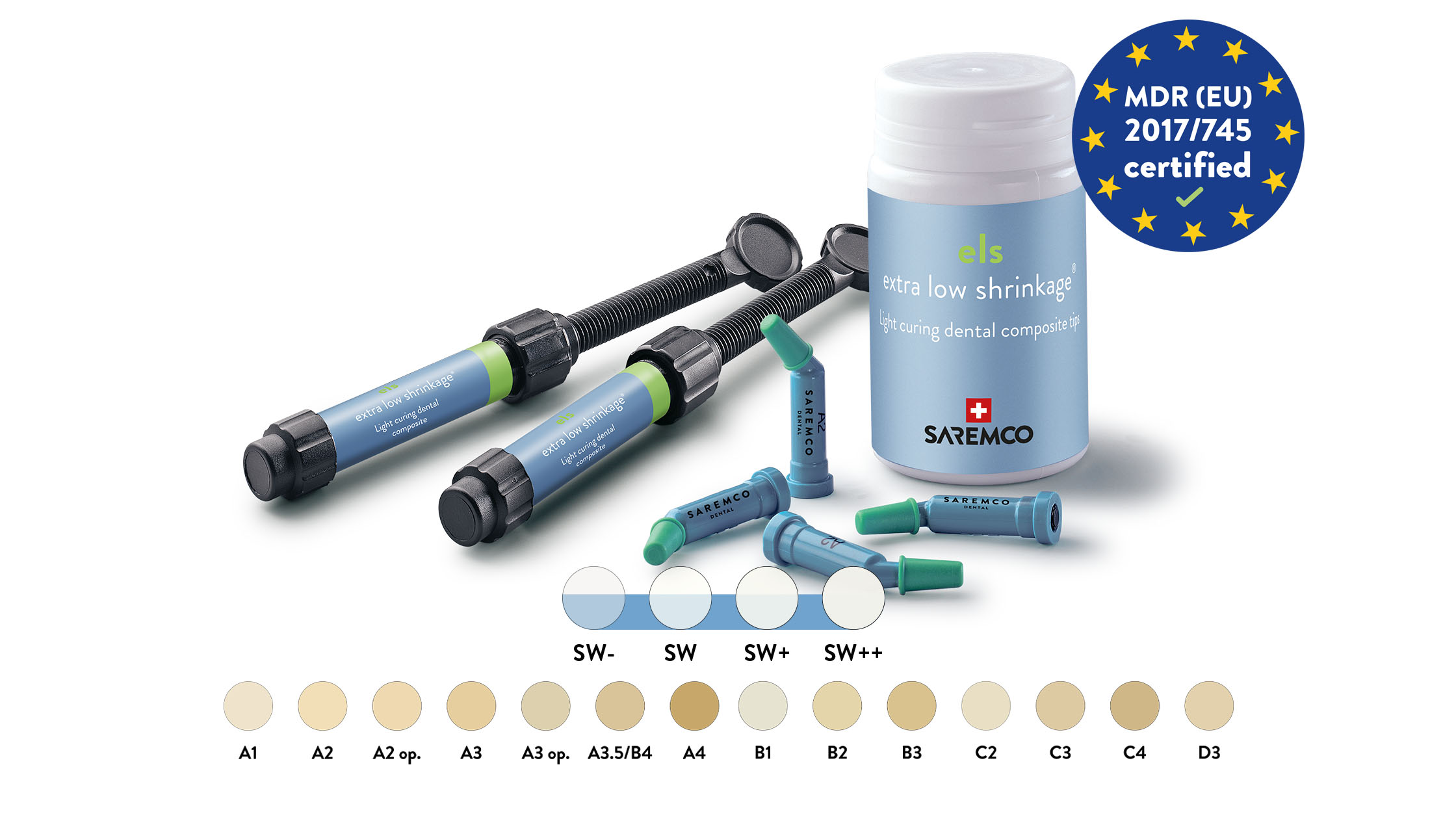
In addition to the MDR certification of the quality management system, SAREMCO Dental AG successfully passed the Medical Device Single Audit Program (MDSAP) at the end of 2023 for the USA, Canada, and Australia. This confirms compliance with the regulatory requirements for these countries. These achievements underscore SAREMCO Dental’s commitment to meeting the highest quality and safety standards and being optimally positioned for future challenges.
“SAREMCO Dental has been committed to the highest quality and innovation in dental materials since its inception,” says Sven Hauser, CEO of SAREMCO Dental AG. “The certifications we have achieved confirm our continuous pursuit of excellence and provide our customers with the assurance that the products they use are clinically proven and fully comply with the latest regulatory requirements.“

Sven Hauser, CEO SAREMCO Dental AG
Franca Schmid, Owner and Board President
SAREMCO Dental AG is a renowned medical technology company based in Rebstein, Switzerland. For over 35 years, the company has been developing and manufacturing high-quality dental products used in dental practices and laboratories worldwide. With a clear focus on quality and safety, SAREMCO Dental continuously contributes to the advancement of dentistry and creates a supportive environment for personal growth and professional development.

